Portable PCR! Testing the miniPCR for DNA sequencing in the field
— This post contributed by Aaron Pomerantz appeared originally on 7/5/2017 in thenextgenscientist.com
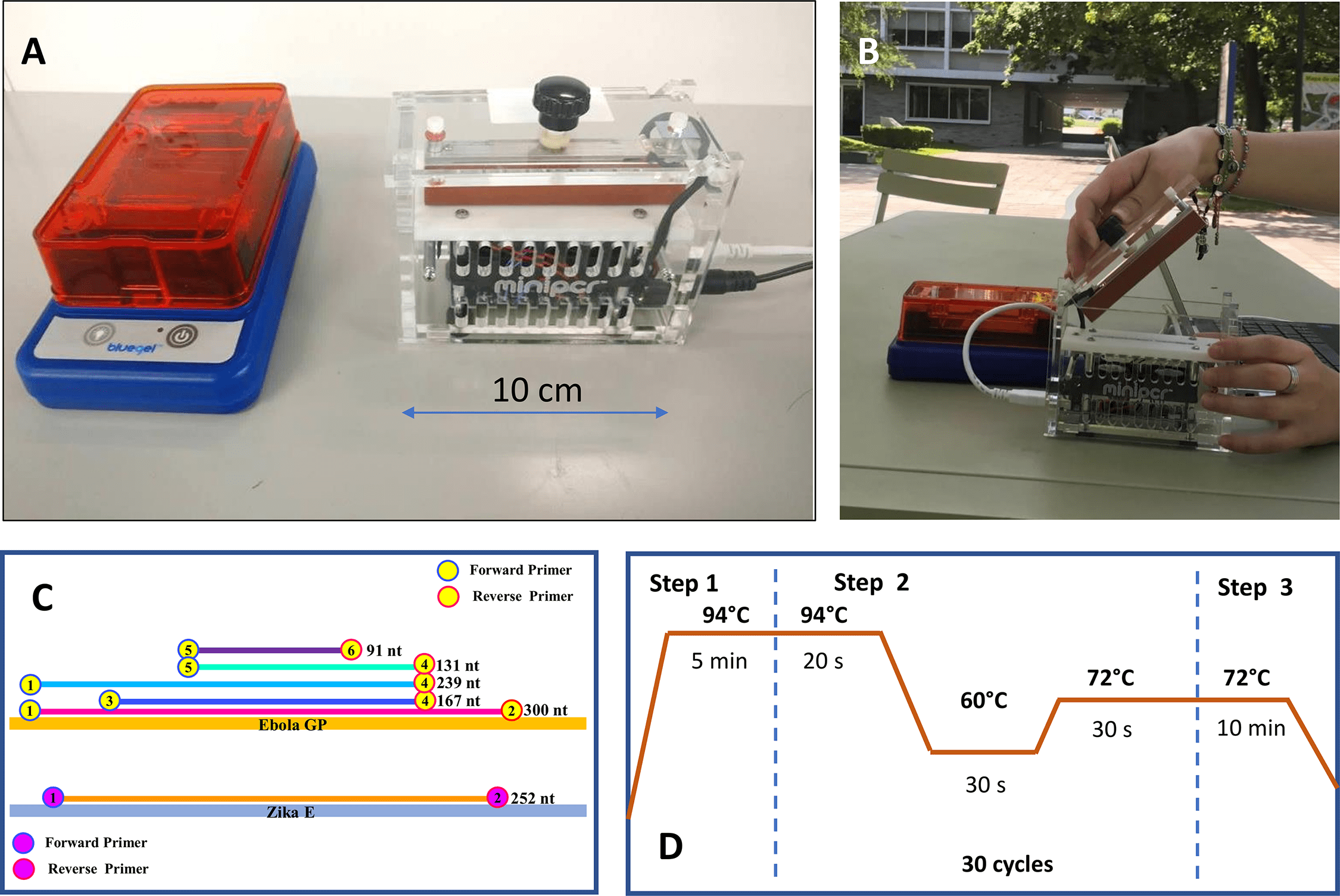
I’ve recently been testing portable tools for a project to take the “lab into the field”. One interesting little piece of equipment I heard about was the miniPCR, a portable thermocycler that has been used for field PCR experiments, for instance “A simple, economical protocol for DNA extraction and amplification where there is no lab“.

I got my hands on one of these little gadgets and put it to the test this week to see if I could amplify genes commonly referred to as DNA “barcodes”, which are used for species identification and molecular phylogenetic trees, such as: “Molecular phylogeny of Atractus (Serpentes, Dipsadidae), with emphasis on Ecuadorian species and the description of three new taxa “. This snake paper published by my colleagues in Ecuador utilized partial genes sequences of 16S, cytb, and ND4 genes to generate the snake phylogeny below:

My goal was to perform an experiment with the same DNA sequences as the snake paper using the miniPCR to determine if we can perform amplification of these genes outside of a lab setting. The end goal of the PCR amplification is to feed these sequences into the Oxford Nanopore Technologies (ONT) MinION, a portable gene sequencing machine.
The Oxford Nanopore MinION has a barcoding kit, which allows you to pool amplicons. Each primer used for amplification needs a special “universal tail” adapted beforehand, so I ordered primers for 16S, cytb, ND4, as well as COI with the ONT primer tails as follows:
5’ TTTCTGTTGGTGCTGATATTGC-[project-specific forward primer sequence] 3’
5’ ACTTGCCTGTCGCTCTATCTTC-[project-specific reverse primer sequence] 3’
So for example, the 16S primers looks like this:
16S_F_ONT: TTTCTGTTGGTGCTGATATTGCCGCCTGTTTAYCAAAAACAT
16S_R_ONT: ACTTGCCTGTCGCTCTATCTTCCCGGTCTGAACTCAGATCACGT
Now with the primers and miniPCR in hand, I just needed some snake DNA! Lucky for me, a graduate student colleague in another lab at UC Berkeley had plenty of snake samples to work with (below referred to as “ALS” and “BRK”), so we extracted DNA using a standard salt extraction protocol.
I whipped up a standard mix for a PCR reaction [10X PCR buffer (5 ul), MgCl2 (2 ul), dNTP (1 ul), H2O (~41 ul), Taq (platinum Taq) (0.5 ul)] using primers for 16S, cytb, ND4 and COI and ran the miniPCR under following settings:

To test the “portability” aspect of the miniPCR, I ran it at my apartment powered by an external Poweradd battery.

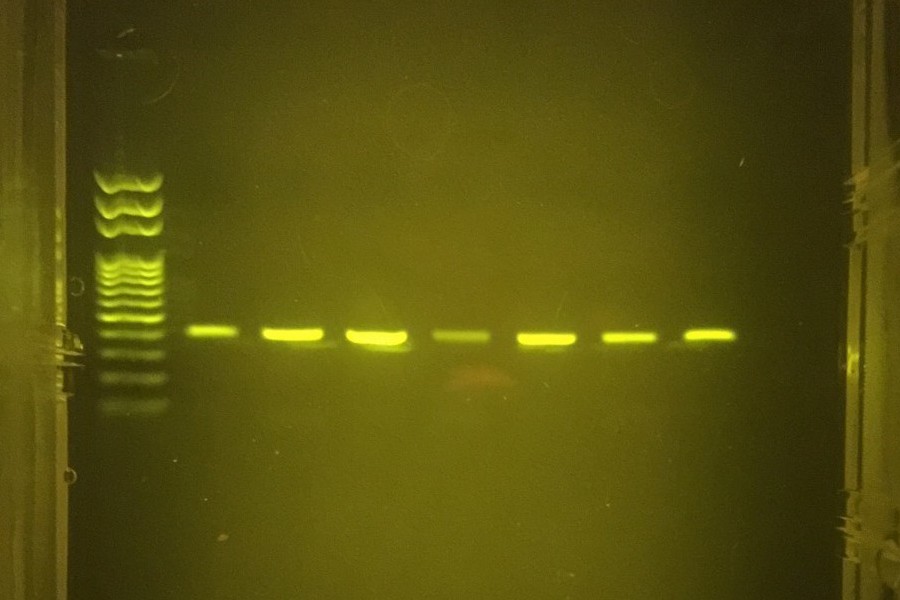
Here are the result of the first PCR run on a gel after a SPRI bead cleanup:
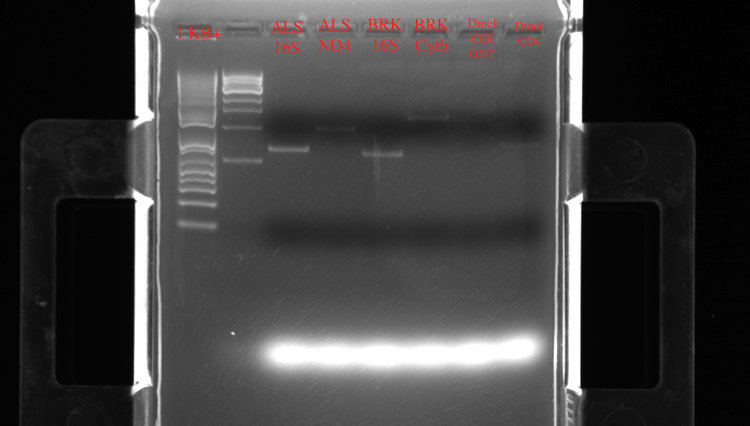
Left to right: two different ladders (1Kb+), ALS 16S (15.8 ng/ul), ALS ND4 (12.6 ng/ul), BRK 16S (10 ng/ul), BRK Cytb (11 ng/ul). The Drosophila (Dmel) COI samples didn’t seem to amplify well.
So not bad with the first round of miniPCR! Overall, the lengths on the gel match the expected amplicon length (16S = 585 bp, ND4 = 901 bp, Cytb = 1209 bp). To play it on the safe side, I also had the amplicons sent off to our DNA sequencing facility on campus and confirmed that they were indeed a match to the expected genes. The exception was the Drosophila sample, which interestingly matched to a Drosophila endosymbiotic bacteria (Wolbachia).
Now for the ONT barcode kit, we need to perform one more round of PCR, this time adding the barcode adapters 1 through 12. I made the scheme below to add barcodes to each individual sample of reptile DNA: ALS and BRK (both reptile species from my colleague at Berkeley) as well as some insect samples Plodia (moth), Junonia (butterfly) from our lab, and a Junonia collected form Peru.
Barcode 1: ALS 16S
Barcode 2: ALS ND4
Barcode 3: BRK 16S
Barcode 4: BRK Cytb
Barcode 5: ALS Cytb
Barcode 6: BRK ND4
Barcode 7: Plodia COI
Barcode 8: Junonia Lab COI
Barcode 9: Junonia Peru COI
Barcode 10: ALS 16S + ALS ND4
Barcode 11: BRK 16S + Junonia Lab COI
Barcode 12: Plodia COI + Junonia Lab COI
The second PCR reaction looked like this: Barcode adapter (2ul), PCR DNA template, H2O (44-47 ul), PCR Master Mix (50 ul) and I let the miniPCR do its thing again:

And some of the gel results:
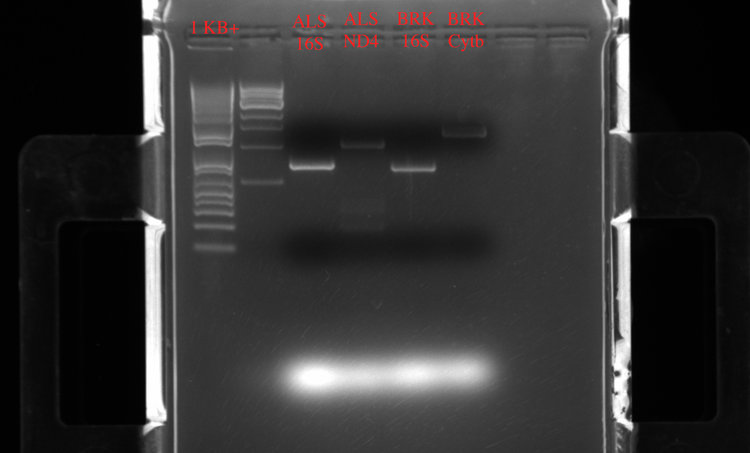
ALS 16S Barcode 1 (152 ng/ul), ALS ND4 Barcode 2 (156 ng/ul), BRK 16s Barcode 3 (168 ng/ul), BRK Cytb Barcode 4 (157 ng/ul)
So overall, I’m impressed with the miniPCR! It has been consistent and reliable in producing expected amplicons – all within the confines of my apartment kitchen! The Poweradd battery works well to make the miniPCR portable, and each run seems to use ~20-30% of the battery.
The next step involves pooling these barcoded amplicons and sequencing on the MinION, which I have recently done but will dedicate a whole post to the sequencing and analysis next. Stay tuned!
-Aaron